Table of Contents
SOLID DIFFUSION
- Compressed source code: Solid Diffusion
- Seminar presentation : solid_diffusion.pdf
- TerraME version: 1.3.0
WHAT IS IT?
Solid Diffusion in TerraMe
This model describes how diffusion occurs between two adjacent solids at it is based on NetLogo Solid Diffusion model http://ccl.northwestern.edu/netlogo/models/SolidDiffusion.
Solid diffusion is material transport by atomic motion, this phenomena is exhaustively studied in fields as materials science, physics, biology, geology, engineering and chemistry.
The main mechanisms for diffusion in solids are:
- Vacancy diffusion
- Interstitial diffusion
- Impurities
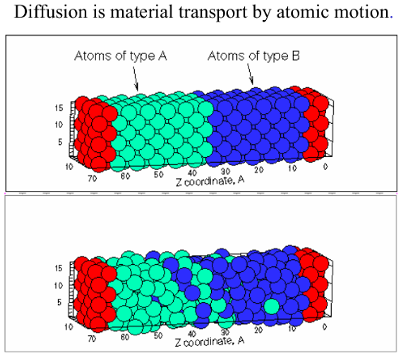
In this model we demonstrate that the Vacancy Diffusion Mechanism is caused by missing atoms in the metal crystal (Vacancies). These vacancies are occupied by atoms that move from areas of high concentration of “Atom of type B” (Figure 1) to areas with low concentration, until the concentration is equal throughtout the sample.
Figure 1. Material transport by atomic motion.
HOW IT WORKS
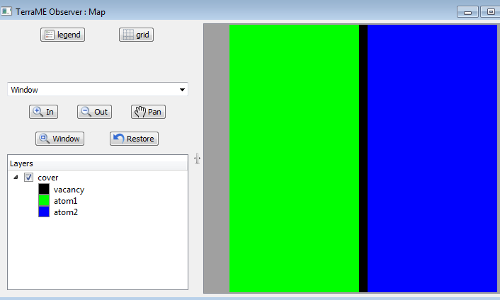
We define an initial state with two types of atoms, “Atoms of type A (green color)” and “Atoms of type B (blue color)”. In the initial state all green atoms are on the left and the blue atoms are on the right side. The vacancies (black color) are assigned to columns between the two materials, Figure 2.
As atoms move into vacancies, the vacancies disperse. In this model for simplification purposes, we assume that the materials have no vacancies in the beginning, and that all the vacancies start off in between the two materials.
Figure 2. Initial state with two types of atoms.
HOW TO USE IT
Parameters:
- SPACE_SIZE: Size of square-shape space. Default value 31 rows by 31 columns – Try 51, 101
- INITIAL_NUMBER Atoms 1: Default value 420
- INITIAL_NUMBER Atoms 2: Default value 420
- INITIAL_NUMBER Vacancies: Default value 60
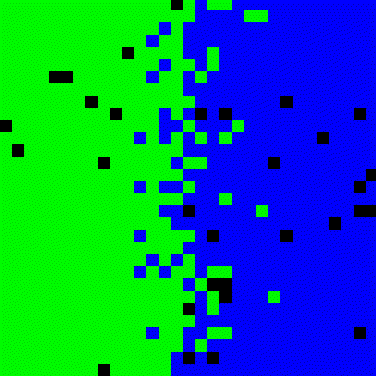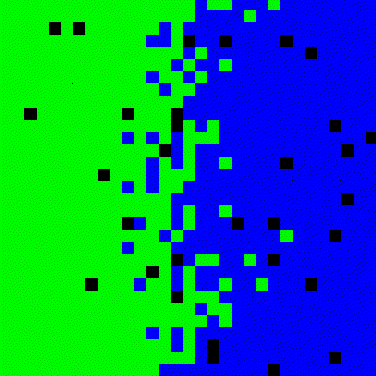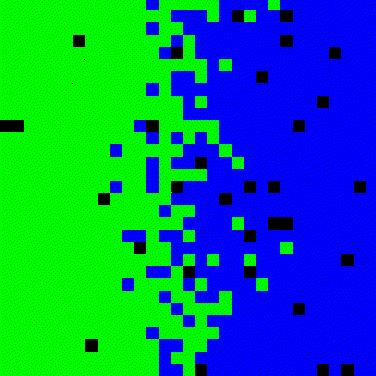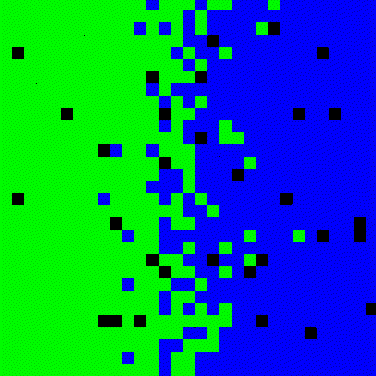
Figure 3. Simulation for two-dimensional diffusion between two solids.
THINGS TO NOTICE
This model is based on Vacancy Diffusion Mechanism, therefore the number of Atoms A and B, as the number of vacancies remains constant over the time. Materials have no vacancies at the beginning.
The model assume equal heat distribution through the metals.
Every particle has a random probability to move.
THINGS TO TRY
Some things to try with the model are:
- Execute the simulation for long time and observe if the particulates will ever become completely diffused.
- Try to increase or reduce the dimensions of the space (numbers of atoms), and observe the behavior.
- Try to increase the number of vacancies.
- Change the neighbors configuration.
EXTENDING THE MODEL
The model can be extended by considering other physical and chemical properties as:
- Temperature: Atomic diffusion occurs during heat treatment, the model could be consider the influence of temperature.
- Atomic size:
- Design of the cellular space according to different crystal structures.
TerraME FEATURES
This model used cellular space, observer, timer and legend.
RELATED MODELS
More information on this model can be found at:
- NetLogo Solid Diffusion. http://ccl.northwestern.edu/netlogo/models/SolidDiffusion
- NetLogo MaterialSim Grain Growth http://ccl.northwestern.edu/netlogo/models/MaterialSimGrainGrowth
- NetLogo GasLab Two Gas http://ccl.northwestern.edu/netlogo/models/GasLabTwoGas
CREDITS AND REFERENCES
- Unordered List ItemPorter, D.A., and Easterling, K.E., Phase Transformations in Metals and Alloys, 2nd ed., Chapman & Hall, 1992
- Unordered List ItemShewmon, P.G., Diffusion in solids, 2nd ed., TMS, 1989
- Wilensky, U. (2007). NetLogo Solid Diffusion model. http://ccl.northwestern.edu/netlogo/models/SolidDiffusion. Center for Connected Learning and Computer-Based Modeling, Northwestern University, Evanston, IL.
- Wilensky, U. (1999). NetLogo. http://ccl.northwestern.edu/netlogo/. Center for Connected Learning and Computer-Based Modeling, Northwestern University, Evanston, IL.